The tumor microenvironment protecting cancer growth
Seeking to fight cancer, science over the past decades has begun to better understand the importance of cells and structures in the immediate vicinity of a tumor. Elucidating the interplay between the tumor micro-environment and cancer itself has also opened new possibilities for medication to overcome this malignant defensive zone.
The abnormal metabolism of cancer cells heavily influences the tumor micro-environment. Most cancer cells rely on aerobic glycolysis as their main source of energy, rather than oxidative phosphorylation. This allows them to increase their intake of glucose, which they use as a carbon source to support rapid proliferation and metastasis.
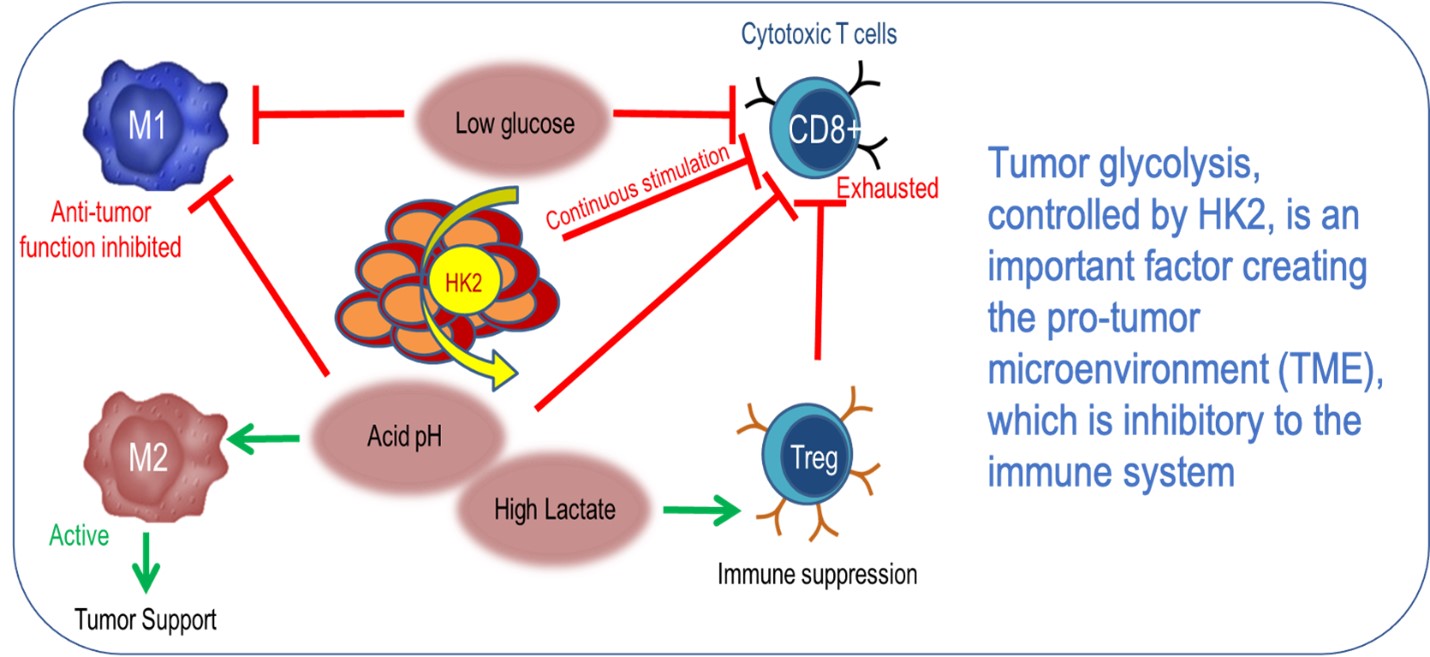
In this process, the cells change glucose to lactate - a phenomenon known as the Warburg effect - which leads to an acidic and low-oxygen micro-environment. This altered chemistry blunts the effectiveness of therapies such as radiation or chemotherapy, not only inside the cancer cells themselves, but also in the tumor micro-environment.
While science is still uncovering new information about the tumor micro-environment, it is clear that this fortress around the tumor plays a role in suppressing the immune system. Elevated levels of lactate support the survival of cancer cells, inhibit the function of tumor-associated macrophages and facilitate the immune evasion of malignant cells.
In the presence of elevated levels of lactate, the macrophages tend to adopt an immunosuppressive phenotype, and collaborate with tumor cells to promote angiogenesis, the formation of blood vessels to supply the tumor with oxygen. In addition, lactic acid suppresses the proliferation of cytotoxic T-lymphocytes by up to 95%.
Targeting the microenvironment to restore immune responses can work, as shown by marketed checkpoint inhibitors. However, the vast majority of cancers are unresponsive to these therapies, and there is a need for new classes of drugs to overcome the protective qualities of the microenvironment. Vidac Pharma’s novel drug candidates, which have shown exciting early results, might bear that promise of a breakthrough solution.
The main culprit – the Hexokinase-2 protein
Vidac Pharma’s drug candidates target the Hexokinase enzyme (HK), which plays a vital role in the abnormal metabolism of cancer cells. The enzyme exists in different isoforms, of which HK1 and HK2 are the most common. In normal adult tissue, HK1 is expressed widely to support glycolysis in harmless situations such as anaerobic exercise, while the expression of HK2 is limited. In contrast, many malignant tissues overexpress HK2.
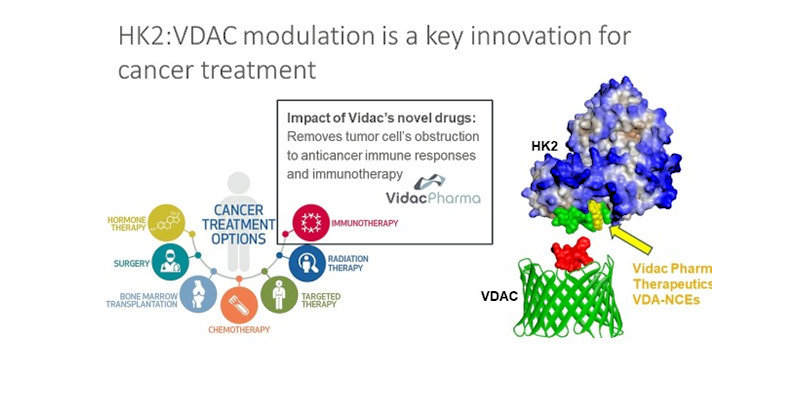
Both HK1 and HK2 attach to the mitochondria – the power plants of the cell – via interaction with the voltage-dependent anion channels (VDAC), which control the passage of many ions and metabolites between the mitochondria and the rest of the cell. The mechanical blockage of the channels by HK2, a very large protein, promotes glycolysis, and thus the production of lactate, with all the tumor-promoting consequences described above. HK2 also blocks the release of molecules which in healthy tissue cause programmed cell death, or apoptosis. This allows the malignant cells to extend their lease on life, which is crucial for a tumor to thrive.
Unblocking the channels: the toposteric effect
Attacking the Warburg effect as a central enabler of cancer has so far yielded no medical results, despite the fact that the phenomenon has been known for a century. That is because glycolysis plays an essential role in healthy cells. Blocking the active site of the HK enzymes would stop glycolysis altogether, and kill the exposed cells.
Vidac Pharma is developing a new paradigm to address this problem. It has booked strong early results with a family of novel chemical entities that stop HK2 from blocking the mitochondrial channels, but do not interrupt its function as a glycolysis catalyst. The company found that certain natural molecules – jasmonates – have the ability to induce a modification of HK2 that detaches it from its VDAC anchor, sending it back into the cytosol, and restoring the cell’s malignant metabolism towards normal.
At Vidac Pharma we call this mechanism of action the toposteric effect. It is an analogy of allosteric regulation, which takes place when molecules bind at specific sites of an enzyme to boost or inhibit its activity. Vidac’s molecules bind to a different site on the enzyme, physically preventing it from attaching to the VDAC channels.
The toposteric effect is Vidac Pharma’s new paradigm for attacking cancer with molecules that do no harm to the rest of the body. It has shown strong first results in vitro and in Phase 2 testing in patients with early carcinoma and a lymphomatic form of skin cancer.

